28
9. Technical data
Notes:
• Do not use the device where it may be exposed to flammable gas.
• This device fulfils the provisions of the EC directive 93/42/EEC (Medical Device
Directive) and the European Standard EN13544-1:2007, Respiratory therapy
equipment - Part1: Nebulizing systems and their components.
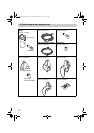
= Type B
= Type B
= Class ll
=
Read the instruction manual carefully
Important information regarding Electro Magnetic Compatibility (EMC)
With the increased number of electronic devices such as PC’s and mobile
(cellular) telephones, medical devices in use may be susceptible to
electromagnetic interference from other devices. Electromagnetic interference
may result in incorrect operation of the medical device and create a potentially
unsafe situation.
Medical devices should also not interfere with other devices.
In order to regulate the requirements for EMC (Electro Magnetic Compatibility)
with the aim to prevent unsafe product situations, the EN60601-1-2 standard
has been implemented. This standard defines the levels of immunity to
electromagnetic interferences as well as maximum levels of electromagnetic
emissions for medical devices.
This medical device manufactured by OMRON Healthcare conforms to this
EN60601-1-2:2007 standard for both immunity and emissions.
Nevertheless, special precautions need to be observed:
• Do not use mobile (cellular) telephones and other devices, which generate
strong electrical or electromagnetic fields, near the medical device. This may
result in incorrect operation of the unit and create a potentially unsafe
situation. Recommendation is to keep a minimum distance of 7 m. Verify
correct operation of the device in case the distance is shorter.
Further documentation in accordance with EN60601-1-2:2007 is available at
OMRON Healthcare Europe at the address mentioned in this instruction
manual.
Documentation is also available at www.omron-healthcare.com.
NE-C30_main.book Page 28 Tuesday, December 13, 2011 12:14 PM