5.3 396R ORP CALIBRATION. An ORP loop is best
calibrated using an ORP standard solution. Most indus-
trial applications have a number of ORP reactions
occurring in sequence or simultaneously. There can be
several components that are oxidized or reduced by
the reagents that are used. Theoretically, the ORP
potential is absolute because it is the result of the oxi-
dation-reduction equilibrium. However, the actual
measured potential is dependent on many factors,
including the condition of the surface of the ORP plat-
inum electrode. Therefore, the sensor should be
allowed 1-2 hours to become “conditioned” to the
stream when first set-up or after being cleaned.
5.3.1 ORP Calibration Procedure
1. Make a temporary electrical connection between
the sensor and the instrument.
2. Obtain a ORP standard solution (PN R508-8oz) or
one can be made quite simply by adding a few
crystals of quinhydrone to either pH 4 or pH 7
buffer. Quinhydrone is only slightly soluble; there-
fore, only a few crystals will be required.
3. Immerse the sensor in the standard solution. Allow
1-2 minutes for the ORP sensor to stabilize.
4. Adjust the standardize control of the instrument to
the solution value shown in Table 5-1. The result-
ing potentials, measured with a clean platinum
electrode and saturated KCl/AgCl reference elec-
trode, should be within ±20 millivolts of the value
shown in Table 5-1. Solution temperature must be
noted to insure accurate interpretation of results.
The ORP value of saturated quinhydrone solution
is not stable over long periods of time. Therefore,
these standards should be made up fresh each
time they are used.
5. Remove the sensor from the buffer, rinse and
install in the process.
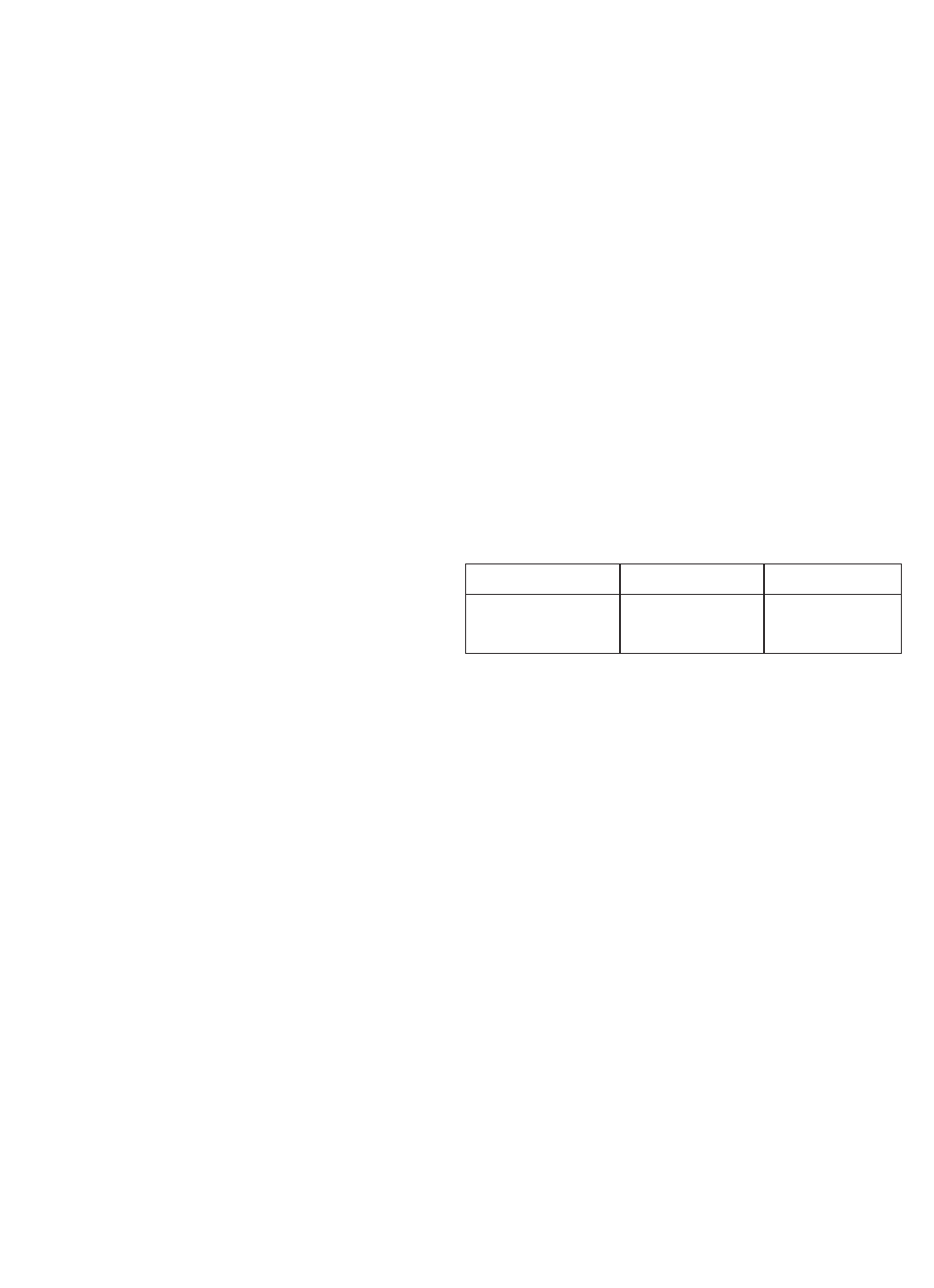
TABLE 5-1
ORP of Saturated Quinhydrone Solution
pH 4 pH 7
TEMPERATURE
°C 20 25 30 20 25 30
Millivolt Potential 268 264 260 94 87 80
MODEL 396R pH/ORP SECTION 5.0
START UP AND CALIBRATION
31


















