MEASURING PRINCIPLE
2 - 9
ETC00781(1) Series 100 e 10/2001
OXYGEN MEASUREMENT (EO
2
ELECTROCHEMICAL PRINCIPLE)
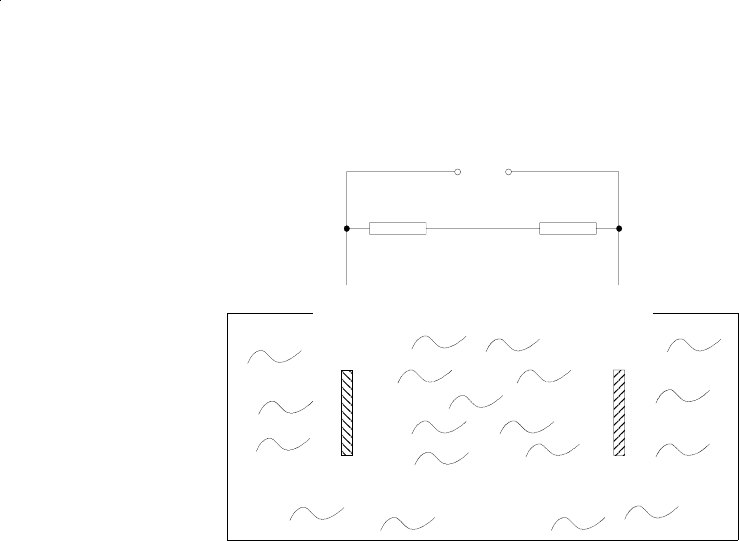
Fig. 2-6: Reaction of galvanic cell
The electric current between the electrodes is proportional to the O
2
concentration in the gas
mixture to be measured. The signals are measured as terminal voltages of the resistor (6) and the
thermistor (5) for temperature compensation.
The change in output voltages (mV) of the senor (11) represents the oxygen concentration.
Note !
Depending on measuring principle the electrochemical O
2
cell needs a minimum internal consumption
of oxygen (residual humidity avoids drying of the cell). Supply cells continuously with dry sample gas
of low grade oxygen concentration or with oxygen-free sample gas could result a reversible detuning
of O
2
sensitivity. The output signal will become instabil.
For correct measurement the cells have to be supplied with O
2
concentrations of at least 0.1 Vol.-%.
We recommend to use the cells in intervall measurement (purge cells with conditioned (dust removal
but no drying) ambient air during measurement breaks).
If it is necessary to interrupt oxygen supply for several hours or days, the cell has to regenerate (supply
cell for about one day with ambient air). Temporary flushing with nitrogen (N
2
) for less than 1 h (e.g.
analyzer zeroing) will have no influence to measuring value.
Resistor (6)
Thermistor (5)
(11)
(Red) (Black)
(+)
Lead-
Anode (1)
(-)
Gold-
Cathode (2)
Summary reaktion O
2
+ 2 Pb ¤ 2 PbO
Electrolyte (3)
(ph 6)
2 Pb + 2 H
2
O ¤ 2 PbO + 4 H
+
+ 4 e
-
O
2
+ 4 H
+
+ 4 e
-
¤ 2 H
2
O


















