X-STREAM
3-11
Instruction Manual
HASAxE-IM-HS
05/2006
Emerson Process Management GmbH & Co. OHG
3 Principles
3-2 Oxygen Measurement
3-2-2 Electrochemical Measurement
This sensor uses the principle of galvanic cells,
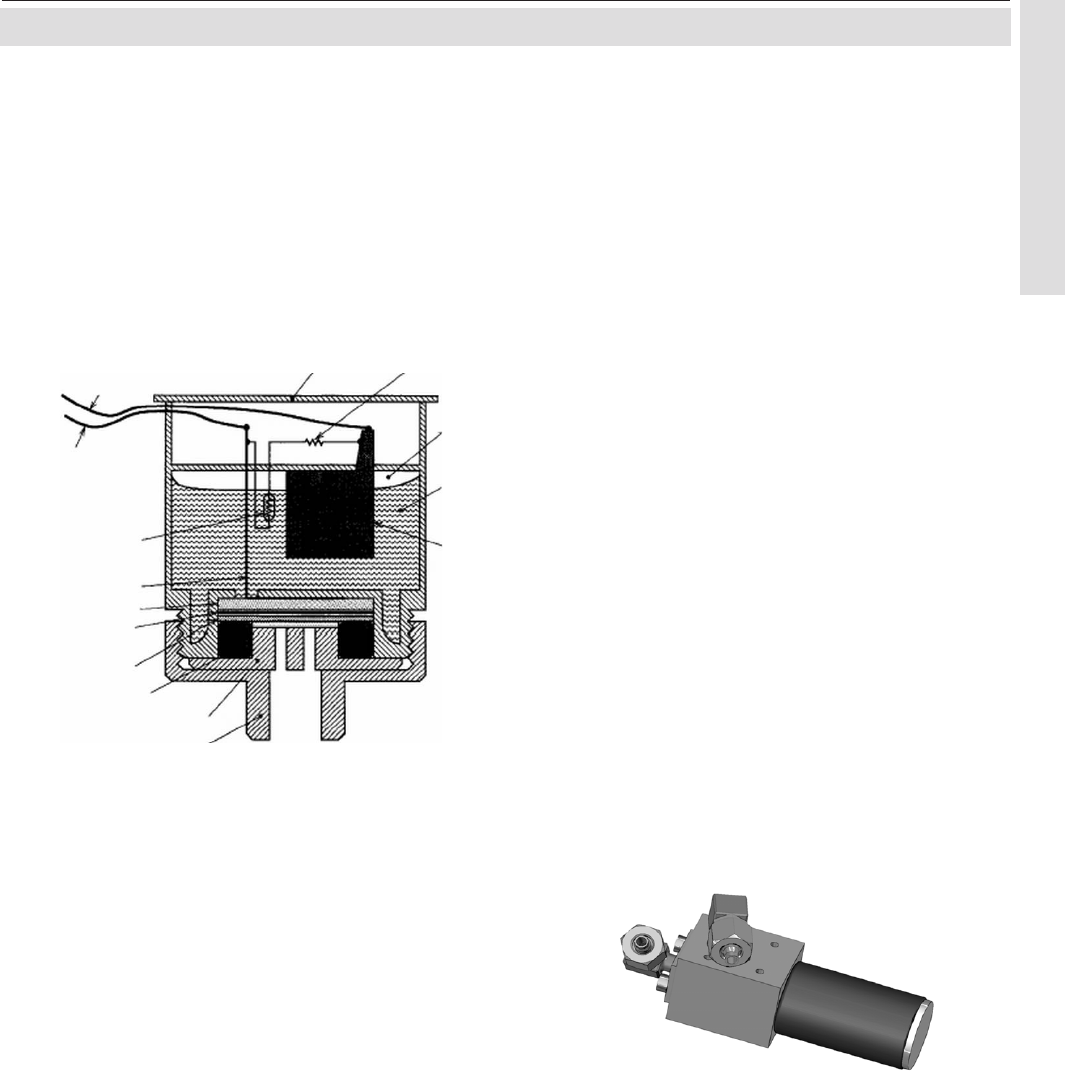
fig. 3-7 shows the design.
Fig. 3-7: Electrochemical Sensor
Assembly Principle
1 Anode (lead)
2 Kathode (Gold)
3 Electrolyte solution
4 Membrane
5 Thermistor
6 Resistance
7 Titanum wire
8 O-Ring
9 Pressure compensating volumes
10 Lid
11 Electrical connections
12 Lids
13 Current collector
12
9
8
4
10
1
6
5
7
3
11
2
12
11
13
The electrochemical oxygen sensor‘s key
components are a lead anode (1) and a gold
cathode (2) surrounded by a special acid
electrolyte (3).
The gold electrode is integrated solid with the
membrane,which is a non-porous fluororesin
membrane. Oxygen which barely diffuses
through the membrane is electrochemically
reduced on the gold electrode.
The temperature compensating thermistor and
adjusting resistance are connected between
the cathode and anode. The current generated
by oxygen reduction is converted into a voltage
by these resistances.
The value of the current flowing to the thermistor
and resistance varies in proportion to the
oxygen concentration of the measuring gases
which contact the membrane. Therefore, the
voltage at the terminal of the resistances is
used for the sensor output to measure the
oxygen concentration.


















